PI: Carine Vergne-Vaxelaire, Anne Zaparucha, Véronique De Berardinis
The hydrolysis of nitriles into the corresponding carboxylic acids usually requires harsh acidic or basic conditions, which can lead to unwanted by-products. Enzymatic hydrolysis by nitrilases is an interesting alternative that tolerates fragile functional groups.
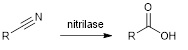
We have extended the diversity of known nitrilases and established their substrate profile.1,2 Particularly robust nitrilases are used immobilised in microfluidic systems (LETI and LITEN collaboration, CEA Grenoble).3,4
This work is supported by ANR funding (BILLIE ANR-22-CE51-0026).
- Vergne-Vaxelaire C, Bordier F., Fossey A., Besnard-Gonnet M., Debard A., Mariage A., Pellouin V., Perret A., Petit J.-L., Stam M., Salanoubat M., Weissenbach J., de Berardinis V., Zaparucha A. ; Nitrilase Activity Screening on Structurally Diverse Substrates: Providing Biocatalytic Tools for Organic Synthesis; Adv. Syn. Catal. 2013, 355, 1763-1779; DOI:10.1002/adsc.201201098
- Bordier F., Stam M., Darii E., Tricot S., Fossey A., Rohault J., Debard A., Mariage A., Pellouin V., Petit J.-L., Perret A., Vallenet D., Salanoubat M., Weissenbach J., Vergne-Vaxelaire C., Zaparucha A.; Large α-aminonitrilase activity screening of nitrilase superfamily members: Access to conversion and enantiospecificity by LC–MS; J. Mol. Cat. B :enzymatic 2014, 107, 79-88; DOI:10.1016/j.molcatb.2014.05.019
- Bessonnet T., Mariage A., Petit J.-L., Pellouin V., Debard A., Zaparucha A., de Berardinis V., Vergne-Vaxelaire C.; Purification, Characterization and Biocatalytic Application of Nitphym, a Robust Thermostable Nitrilase from Paraburkholderia phymatum“ Front.Bioeng. Biotech. 2021, 9:686362; DOI:10.3389/fbioe.2021.686362
- Teepakorn C., P. Zajkoska, Cwicklinski G., Roux J.-M., De Berardinis V., Zaparucha A., Nonglaton G., Anxionnaz-Minvielle Z.; Nitrilase immobilization screening for nicotinic acid production developments from micro-scale batch to continuous process; Biotechnol J. 2021, 16, 2100010; DOI:10.1002/biot.202100010.