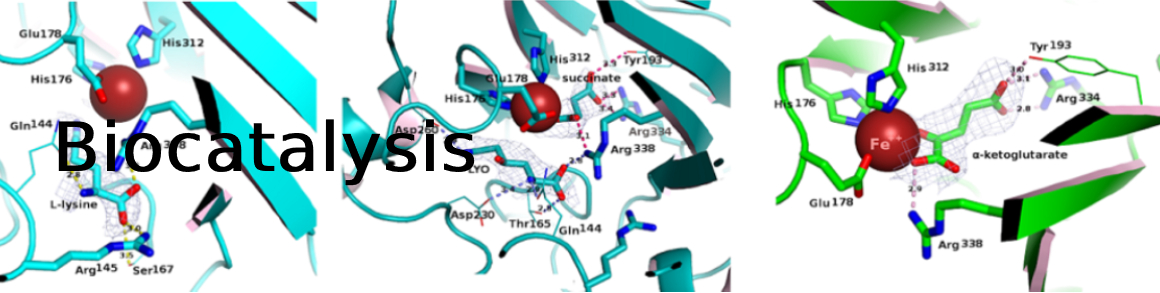
PI: Anne Zaparucha
CoA ligases (EC 6.2.1.X) are ATP-dependent enzymes which catalyse the adenylation of carboxylic acids, followed by the conversion of the acyl adenylate to the coenzyme A thioester. The native reaction can be diverted by adding an external nucleophile, such as an amine, to the acyl adenylate to form the corresponding amide.
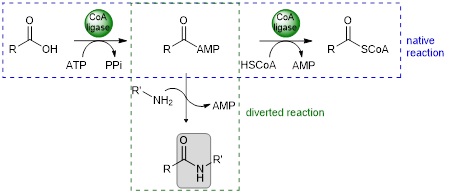
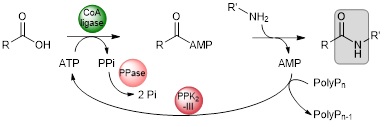
Non-catalysed addition of the external nucleophile is favoured at high temperature. To set up a onepot chemo-enzymatic cascade, we need thermophilic CoA ligases capable of withstanding the temperature of the reaction medium. We have therefore built up a collection of thermophilic CoA ligases. In parallel, we have developed a system for recycling ATP through the combined action of a PPK2-III polyphosphate kinase and a pyrophosphatase.1
After focusing on the synthesis of naked lactams, a subject on which we have established a collaboration with D.B. Janssen and A.-M. Thunnissen (University of Groningen, NLD), we are now working on the synthesis of functionalized lactams.
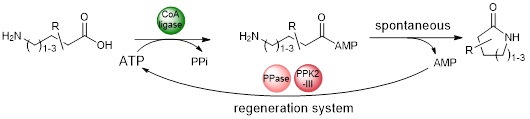
This theme is being pursued through the ANR SMALA, of which we are coordinators (ANR-21-CE07-0061).
- Lelièvre C. M., Balandras M., Petit J.-L., Vergne-Vaxelaire C., Zaparucha A., ATP regeneration system in chemoenzymatic amide bond formation with thermophilic CoA ligase; ChemCatChem 2020, 12, 1184-1192; DOI:10.1002/cctc.201901870