Baud D., Peruch O., Saaidi P.-L., Fossey A., Mariage A., Petit J.-L., Salanoubat M., Vergne-Vaxelaire C., de Berardinis V., Zaparucha A.
Adv. Syn. Catal. 2017, 359, 1563-1569
Abstract
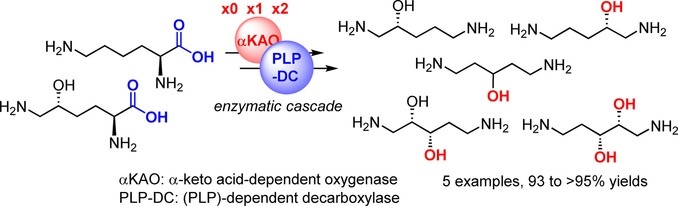
Amino alcohols are a very common structural motif in natural and synthetic molecules. Starting from l-lysine and hydroxy-l-lysine, a straightforward biocatalytic synthesis of beta- and gamma-amino alcohols is presented. Diastereoselective C–H oxidation catalyzed by an alpha-keto acid-dependent oxygenase followed by cleavage of the carboxylic acid moiety of the corresponding chiral hydroxy amino acid by a pyridoxal phosphate-dependent decarboxylase enabled the formation of the target amino alcohols with moderate to complete conversions. Four beta- and gamma-amino alcohols were obtained on a small scale in excellent yields and stereoselectivities.