Mayol O., David S., Darii E., Debard A., Mariage A., Pellouin V., Petit JL., Salanoubat M., de Berardinis V., Zaparucha A., Vergne-Vaxelaire C.
Catal. Sc. Technol. 2016, 6, 7421-7428
Abstract
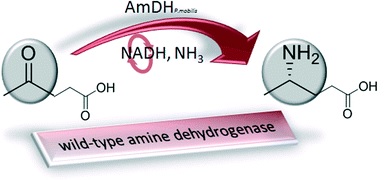
The biocatalytic reductive amination of ketone to chiral amine is one of the most challenging reactions. Using a genome-mining approach, we found proteins catalyzing the reductive amination of ketones without a carboxylic function in the α or β position. The synthesis of (4S)-4-aminopentanoic acid (ee ≥99.5%) was achieved with the thermoactive amine dehydrogenase (AmDH) AmDH4 from Petrotoga mobilis in 88% yield. The high stability and substrate tolerance make AmDH4 a very good starting point for further discovery of reductive amination biocatalysts with an enlarged substrate range. This is the first report of wild-type enzymes with related genes having proper NAD(P)H–AmDH activity.