Bordier F., Stam M., Darii E., Tricot S., Fossey A., Rohault J., Debard A., Mariage A., Pellouin V., Petit J.-L., Perret A., Vallenet D., Salanoubat M., Weissenbach J., Vergne-Vaxelaire C., Zaparucha A.
J. Mol. Cat. B :enzymatic 2014, 107, 79-88
Abstract
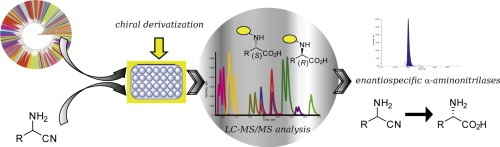
A high-throughput screening for the identification of nitrilases demonstrating activity towards alpha-aminonitriles is reported. A LC–MS assay giving access to both conversion and enantiospecificity was developed. 588 candidate enzymes were screened as cell lysates against six alpha-aminonitriles in 96-well microplates. The candidate enzymes were selected following two criteria, their sequence identity with a set of known nitrilases or their phylogenetic position among the nitrilase superfamily. Five enzymes were identified and found to hydrolyse alpha-aminonitrile into the corresponding alpha-aminoacid. The substrate range was found to be very narrow as only two different alpha-aminonitriles, 2-aminovaleronitrile and 2-amino-2-phenylacetonitrile, were found to be substrates. The biocatalytic capabilities of three enzymes were further investigated and the best result was obtained with an enzyme from Burkholderia xenovorans catalysing the enantiospecific hydrolysis of 2-aminovaleronitrile into (S)-norvaline with excellent conversion and enantiomeric excess.